
While chemotherapy can stimulate antitumor immunity, it may also impair immune cell function and induce lymphodepletion, reducing effectiveness of subsequent immunotherapy. Thus, nanoparticle-based drug delivery systems have been developed for their improved drug biodistribution and reduced toxicity, striking a delicate balance between efficacy and safety. However, the interaction between nanoparticles and immune system, particularly the formation of protein corona on nanoparticle surfaces, remains unclear.
ApoA-1, a natural ligand of scavenger receptor class B type 1 (SR-B1), is commonly enriched in protein corona of lipid nanoparticles and plays crucial roles in modulating nanoparticle-cell interactions. High-density lipoprotein (HDL) is a natural nanoparticle containing ApoA-1, and it mainly transports hydrophobic cargo directly into the cytosol in an endocytosis-independent pathway via binding with SR-B1, while ApoA-1 remains in circulation and finally undergoes degradation in the kidney. Recently, synthetic HDLs (sHDLs) composed of ApoA-1 mimic peptides have been explored, resembling natural HDL in biocompatibility and SR-B1-based cell specificity. Though of different origins, both sHDLs and natural HDLs show capability of regulating innate immune responses.
In a study published in Science Advances, a research team led by ZHANG Pengcheng from ShanghaiTech University, LI Yaping from the Shanghai Institute of Materia Medica of the Chinese Academy of Sciences, and WANG Hao from China State Institute of Pharmaceutical Industry, revealed that ApoA-1 can transport drugs encapsulated within lipid nanoparticles to myeloid cells, leading to myelopenia and consequently lymphopenia, and demonstrated that these side effects can largely be avoided simply by conjugating drugs to the nanoparticles without reducing antitumor activity of drugs.
To investigate the influence of ApoA-1 on the immune response, researchers created two variants of maytansine (DM1)-loaded synthetic high-density lipoproteins (D-sHDL) with DM1 either physically entrapped (ED-sHDL) or chemically conjugated to ApoA-1 (CD-sHDL). By comparing their cell uptake mechanism, biodistribution, in vivo efficacy and safety, they found that CD-sHDL showed less accumulation in the tumor-draining lymph nodes (DLNs) and femur. This resulted in lower toxicity against myeloid cells than ED-sHDL via avoiding SR-B1-mediated DM1 transportation into granulocyte-monocyte progenitors and dendritic cells.
Therefore, higher densities of lymphocytes in tumors, DLNs, and blood were recorded in mice receiving CD-sHDL, leading to better efficacy and immune memory against colon cancer. Furthermore, liposomes with conjugated DM1 (CD-Lipo) showed lower immunotoxicity than those with entrapped drugs (ED-Lipo) through the same mechanism after the opsonization of ApoA-1 in protein corona, indicating the universality of the immunotoxicity alleviation by chemical conjugation.
Given high translational potential of the two investigated lipid nanoparticles (sHDL and liposome) and prevalence of ApoA-1 as a main component of the protein corona, these findings shed light on designing nanoparticle-based chemotherapy strategies by elucidating the role of ApoA-1 in modulating immune responses.
This study deepens the understanding of immune interaction of nanoparticles. Figuring out the complex interplay between nanoparticles and immune system will pave the way for the development of more effective and less toxic cancer therapies, and will impact on the clinical translation of nanoparticles for immune manipulation.
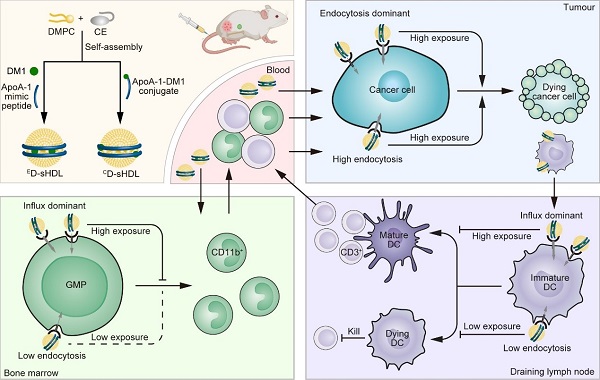
Schematic illustration of sHDL preparation and different impact on toxicity and efficacy of drug loading patterns in sHDL. (Image by ZHENG Chao)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

